If you are reading this, it is quite possible that you, your child, or another family member has been through a clinical trial. Or, you have been involved as a researcher producing preclinical data leading up to such trials. More than a year has gone by since the completion of the study, and you would like to know what the outcome of the trial was. Will the drugs used in the study be made available to the public? Most importantly, will sufficient data resulting from the trials be presented in a timely fashion such that other researchers can learn from them?
ClinicalTrials.gov
In the U.S., one of the first places you might turn to find clinical trial results is the federal website, ClinicalTrials.gov. As summarized on the site itself:
“ClinicalTrials.gov was created as a result of the Food and Drug Administration Modernization Act of 1997 (FDAMA). FDAMA required the U.S. Department of Health and Human Services, through NIH, to establish a registry of clinical trials information”.
Furthermore, the trials which appear on ClinicalTrials.gov (abbreviated in this post as “CTgov”) include any for which an investigational new drug application was filed with FDA:
“for both federally and privately funded trials conducted under investigational new drug (IND) applications to test the effectiveness of experimental drugs for serious or life-threatening diseases or conditions. NIH and the Food and Drug Administration (FDA) worked together to develop the site, which was made available to the public in February 2000.”
Most importantly, U.S. law requires submission of “certain” clinical trial results to the CTgov database:
“The ClinicalTrials.gov registration requirements were expanded after Congress passed the FDA Amendments Act of 2007 (FDAAA). Section 801 of FDAAA (FDAAA 801) requires more types of trials to be registered and additional trial registration information to be submitted. The law also requires the submission of results for certain trials. This led to the development of the ClinicalTrials.gov results database, which contains information on study participants and a summary of study outcomes, including adverse events.
The results database was made available to the public in September 2008. FDAAA 801 also established penalties for failing to register or submit the results of trials.”
(https://clinicaltrials.gov/ct2/about-site/background; with emphasis added.)
Fragile X clinical trial results on ClinicalTrials.gov
For purposes of illustration, (and considering the interests of readers), we’ll examine some clinical trials of pharmaceuticals for the treatment of Fragile X Syndrome.
If we search ClinicalTrials.gov for the term “Fragile X” without quotes (search terms without quotes in the future will be shown between angle brackets, e.g. >search term<), we find 53 trials listed (https://clinicaltrials.gov/ct2/results?term=Fragile+X&Search=Search on April 15, 2015).
By clicking the link, “Modify this search”, we can specify under the “Recruitment” menu only those trials which are completed. The results show 28 studies. (The exclude unknown status checkbox can also be employed, but in this case it has no effect.) No trial results need be posted within one year after the formal completion of a clinical trial (details in the next post), so we will narrow the search results further (using the “Show display options” link/”study completion”) to those trials completed more than one year ago (19) and more than three years ago (12).
The search among completed studies can be further modified to show only those that have results. From that we find only three studies:
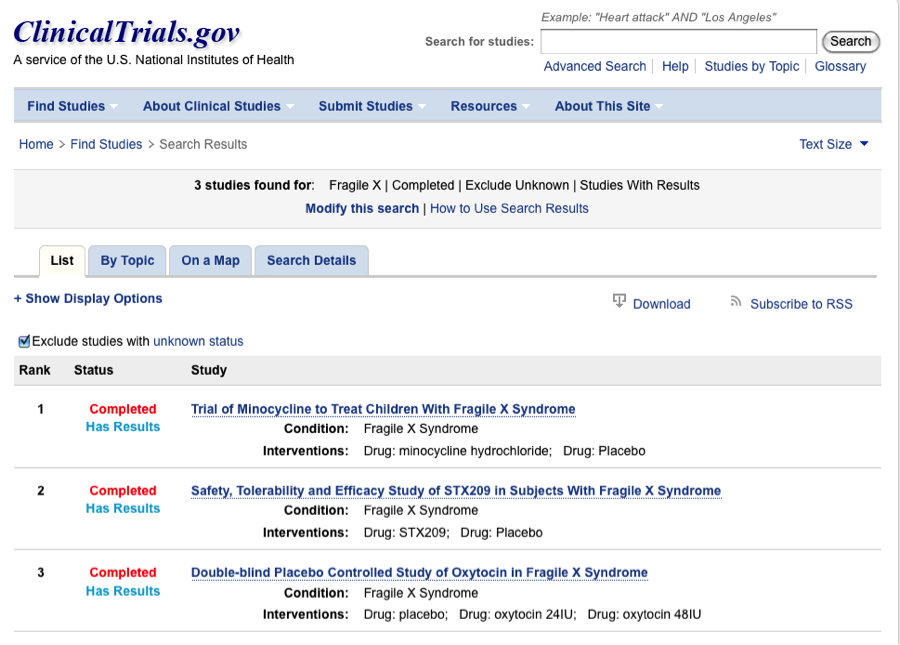
These three trials should present some idea of the information that can be found in the CTgov results database, so we’ll briefly examine them here.
First, many completed clinical trials are not presenting data in a timely fashion. Even three years after a study’s completion, only 25% of completed Fragile X trials have results posted (3/12; see preceding). In the next post we’ll delve into laws in the U.S. and abroad to determine under what circumstances it might be possible to obtain results from completed trials even if delays or waivers are granted to the sponsors, but for now the quality of results that are available on CTgov can be examined.
Fragile X clinical trial results posted on ClinicalTrials.gov
1. Trial of Minocycline to Treat Children With Fragile X Syndrome
ClinicalTrials.gov Identifier: NCT01053156
Official Title: Randomized Double-Blind Controlled Cross Over Trial of Minocycline in Children With Fragile X Syndrome
Sponsor: University of California, Davis
Number Enrolled: 66
Primary Completion Date: December 2011 [Final data collection date for primary outcome measure]
Results First Received: February 8, 2013 [just over 1 year after completion]
One of the most important pieces of information to assess in terms of whether data will be available is the primary trial completion date, which is defined as date on which the final primary outcome measure was made for the final subject. No results need to be submitted for presentation on CTgov prior to one year after the final patient is evaluated for the primary outcome measure. For the minocycline trial, the primary completion date was December 2011. The results were first received in February 2013, just a year after the completion date of the trial, which is very timely.
The minocycline trial was placebo-controlled and blind, meaning it was intended that no one knew whether they were receiving the drug or placebo. It was also a crossover trial, such that each participant received a period of drug and a period of placebo. In other words, each trial subject could act as his or her own control, which can increase the power to detect drug effects.
A typical result table from the Fragile X minocycline trial:
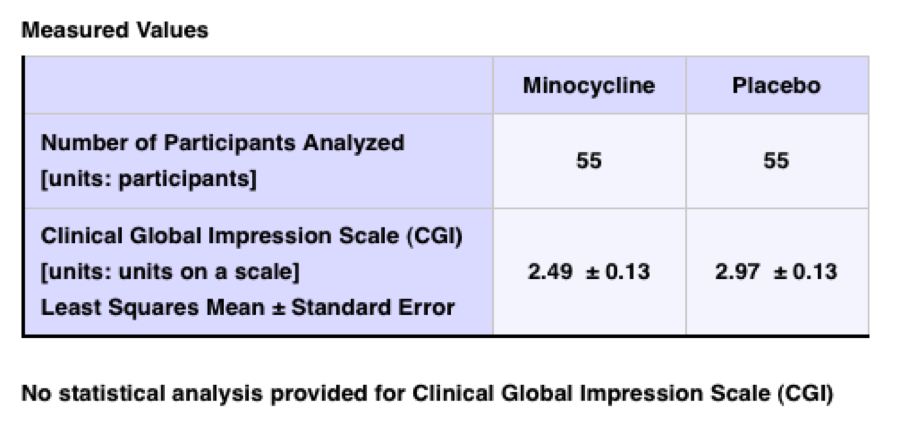
No statistical analysis was provided for the results presented. Nevertheless, since the lay public is intended as an important part of the CTgov audience, a statement interpreting the statistics would be useful. For example, adding or subtracting confidence intervals (the number after the ±) from the average response (the numbers before the ±) to bring them as close as possible, shows no overlap in the results (2.62 v. 2.84). The lack of overlap in what are termed the statistical confidence intervals indicates that the results are significantly different.
An explicit statement as to the basis and strength of that difference would also be useful to know. From the results presented, it might be possible to estimate the strength or “effect size” by looking at the difference of the means, or the difference between the means as a percentage of the test scale. Nevertheless, there are methods to convey effect size to lay people, such as by using the “common language effect size” which is “designed to communicate the meaning of an effect size in plain English, so that those with little statistics background can grasp the meaning.” [https://en.wikipedia.org/wiki/Effect_size; emphasis added.].
For a different test result, a large placebo effect is noted:
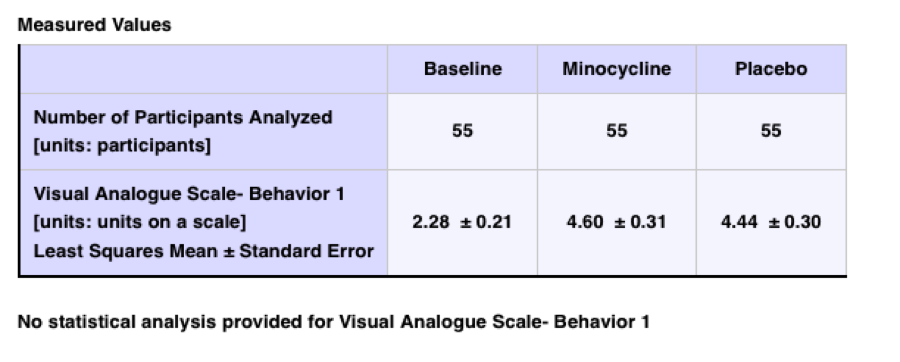
Nevertheless, despite an overlap in the confidence intervals (the placebo effect overlaps the treatment mean 4.74 vs. 4.60), it could have been pointed out that:
“If two statistics have non-overlapping confidence intervals, they are necessarily significantly different but if they have overlapping confidence intervals, it is not necessarily true that they are not significantly different.”
(https://www.cscu.cornell.edu/news/statnews/stnews73.pdf; emphasis added)
Clearly, it would be helpful to have some statement of significance!
Additional potentially significant results were shown for this trial:
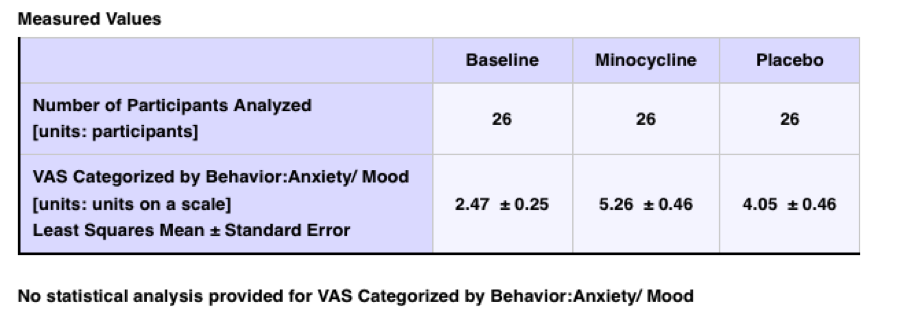
Therefore, there is no actual statement on CT gov characterizing whether the study was considered successful in any way or not. There was a link to a publication on the results page: “Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model”, but that was a preclinical study published in 2009 that was actually used as a basis for the above clinical trial.
2. Safety, Tolerability and Efficacy Study of STX209 in Subjects With Fragile X Syndrome
ClinicalTrials.gov Identifier: NCT00788073
Official Title: A Double-Blind, Placebo-Controlled, Crossover, Flexible-Dose Evaluation of the Efficacy, Safety and Tolerability of STX209 in the Treatment of Irritability in Subjects With Fragile X Syndrome
Sponsor: Seaside Therapeutics, Inc.
Number Enrolled: 63
Primary Completion: March 2010
Results First Received: February 2013 [just under three years after study completion]
In this study, we find a more detailed presentation of statistical results information:
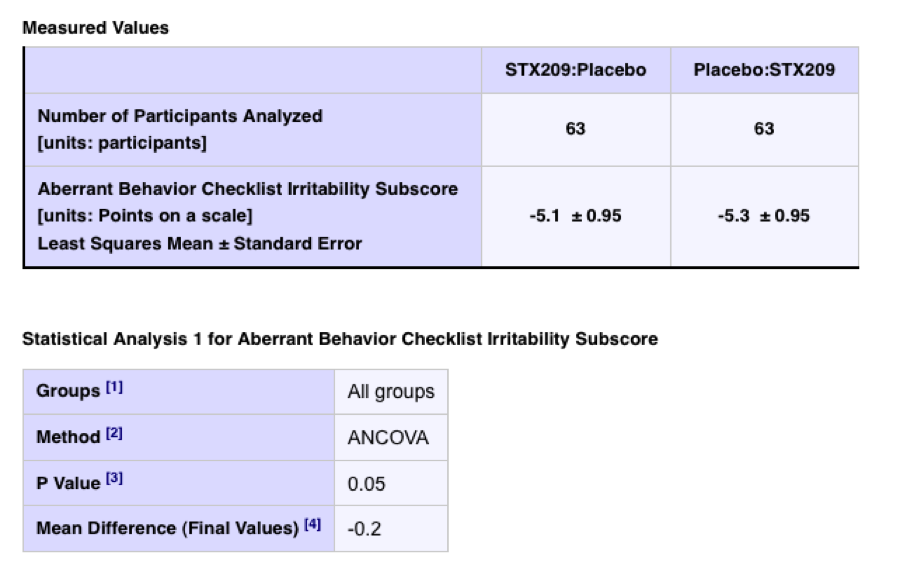
In the absence of a statement or link to a relevant publication from the trial sponsors and investigators, the reader is left to wonder whether the statistical effects noted are considered notable. For example, in the same trial for an analysis of a more specific measure what seems to be a result of obvious statistical significance is presented:

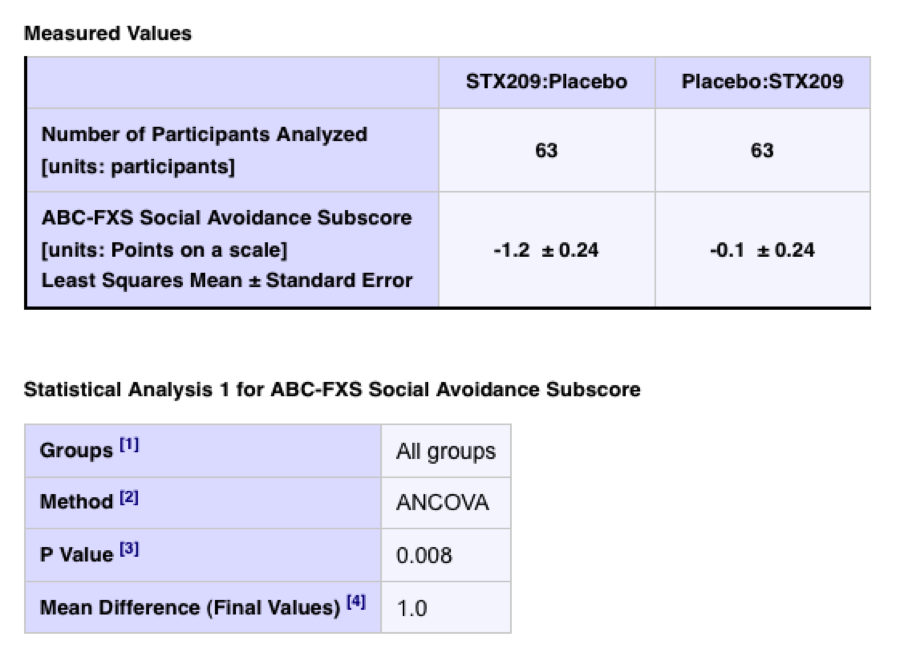
Therefore, avoidance behavior may have declined – a worthy outcome for Fragile X and autistic disorders – but perhaps the overall effect was not so large, i.e. about 1 on a scale of 1 to 12.
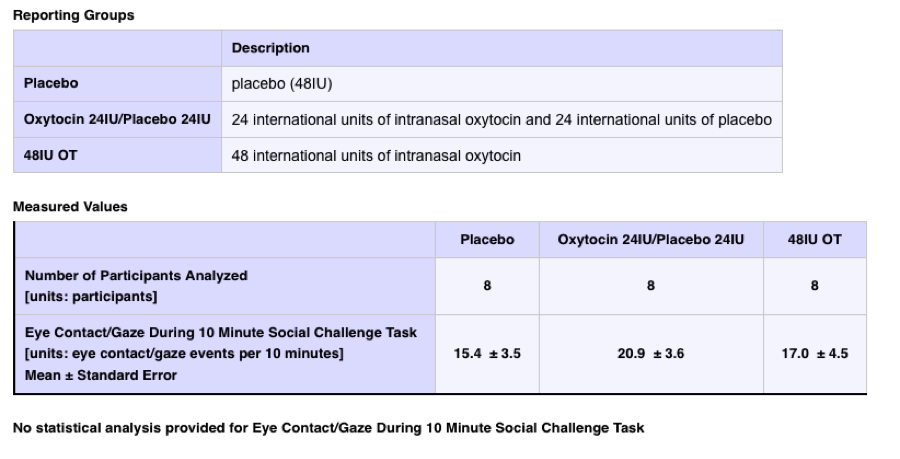
3. Double-blind Placebo Controlled Study of Oxytocin in Fragile X Syndrome
ClinicalTrials.gov Identifier: NCT01254045
Official Title: Double-blind Placebo Controlled Study of Oxytocin in Fragile X Syndrome
Sponsor: Stanford University
Number Enrolled: 10
Primary Completion Date: November 2009
Results First Received: August 2011 [over 1.5 years after study completion]