Fraud Investigation Blog
These arrows can be used to move through posts.
Latest Posts
Assisting litigants with attorney fraud concerns
2025: Robert Bauchwitz is the leader of the ACFE Interest Group on Assisting litigants with attorney fraud concerns. Posts on this topic can be found here.

Recommended Modifications to the 2005 Public Health Service Policies on Research Misconduct, 42 C.F.R. Part 93
The following are suggested modifications to the Code of Federal Regulations of the USA (42 CFR part 93), which deals with the handling of research misconduct. These recommendations were made on October 31, 2022, in response to a Request for Information (RFI) by the federal Department of Health and Human Services’ Office of the Secretary. […]

In memoriam: The federal government should never leave whistleblowers to their own fates against much more powerful employers.
“In 1998, an Alaska Airlines mechanic named John Liotine, who worked in the Alaska Airlines maintenance center in Oakland, California, told the Federal Aviation Administration that supervisors were approving records of maintenance that they were not allowed to approve or that indicated work had been completed when, in fact, it had not.” (Wikipedia: Alaska Airlines […]

The $25,000 sore throat test: list price permissiveness as a possible racket
We can try to think about this sort of extreme healthcare list price tactic from the point of view of fraud and it’s deterrence …

Comment to the Subcommittee on Research and Technology, Committee on Science, Space, and Technology, 115th Congress
1. It is important to obtain accurate assessments of the actual amount of research misconduct Members of Congress, especially members of the Committee on Science, Space, and Technology, should be urged to reconsider the quality of the data being relied upon for the number of research misconduct findings. In an article published in Science Magazine […]

U.S. Supreme Court Petition re Judicial Oversight
[pdf-embedder url=”https://amerares.com/blogs/wp-content/uploads/2017/04/SCt-Cert-Petition-Cropped-040617-FINAL-copy.pdf” title=”SCt Cert Petition – Cropped 040617 – FINAL copy”] A pdf version of the petition can be downloaded here. A text version follows. No.________ ================================================================ In The Supreme Court of the United States ——————————––♦——————————–– UNITED STATES OF AMERICA EX REL. ROBERT P. BAUCHWITZ, M.D., PH.D., Petitioner, v. WILLIAM K. HOLLOMAN, […]

More on research misconduct oversight and conflicts of interest
Background We stated in a recent article discussing the federal Office of Research Integrity (ORI) that, were they to actually act as a real audit entity, then their also acting as a consulting function for the same institutions could produce a conflict of interest (COI). (“The Essential Need for Research Misconduct Allegation Audits”, Science and […]

Research misconduct audit oversight article published in Science and Engineering Ethics
Our manuscript on the importance of employing professional audit principles in the oversight of the U.S. research misconduct investigations, which we originally published in PeerJ Preprints in December 2015, has now been published by Science and Engineering Ethics (“SEE”), a Springer journal. The core of the article remained unchanged from the preprint. At the request […]

July 30 is National Whistleblower Day
The first was celebrated in the U.S. Congress on July 30, 2015. For annually updated details see here.
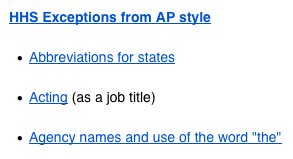
The Grammar and Use of Initialisms by the U.S. Federal Government
This research on the use of initialisms by the United States federal government was produced during our publication of a paper in the Springer journal, Science and Engineering Ethics. The editor of that journal thought it would be worth publishing this information separately, for the benefit of others. We agreed; the following summarizes what we […]

One-Eyed Jacks in the Research Misconduct Universe
Below we reproduce a comment by Albert Donnay (University of Maryland, Baltimore) about the fraud facilitating influence of those in the academic world who have some ethical obligation, if not a duty, to respond to allegations of research misconduct, but for their own self-interests instead turn a blind eye. Dr. Donnay’s comment is particularly interesting […]
Implications of the Feldheim Eaton NSF research misconduct case
The following comment was published January 10, 2016 on Retraction Watch website. (The original can be found here.) It is important because it adds an additional example of the government research misconduct functions which we discussed in our manuscript, “The Essential Need for Research Misconduct Allegation Audits”, published in December 2015. Journalist Joseph Neff noted in […]





